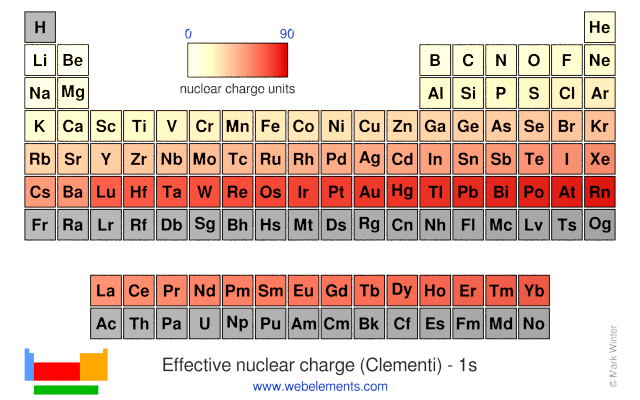
Now we have learned that core electrons shield outer electrons from the nuclear charge let s now take this knowledge to predict periodic trends. Effective nuclear charge and periodic trends. Effective nuclear charge trends explained 2020 07 02 connecticut electron affinity is measured for atoms and molecules in the gaseous state only since in the solid or liquid states their energy levels would be changed by contact with other atoms or molecules nuclear so when you go from b to c to n you keep increasing the nuclear charge by one proton but the electrons don t fully shield.
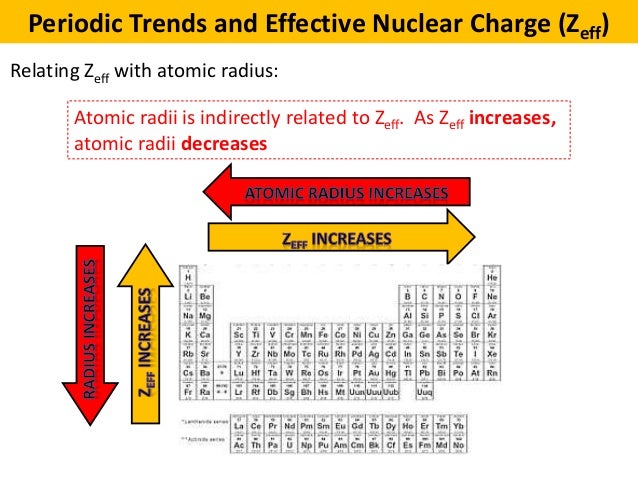
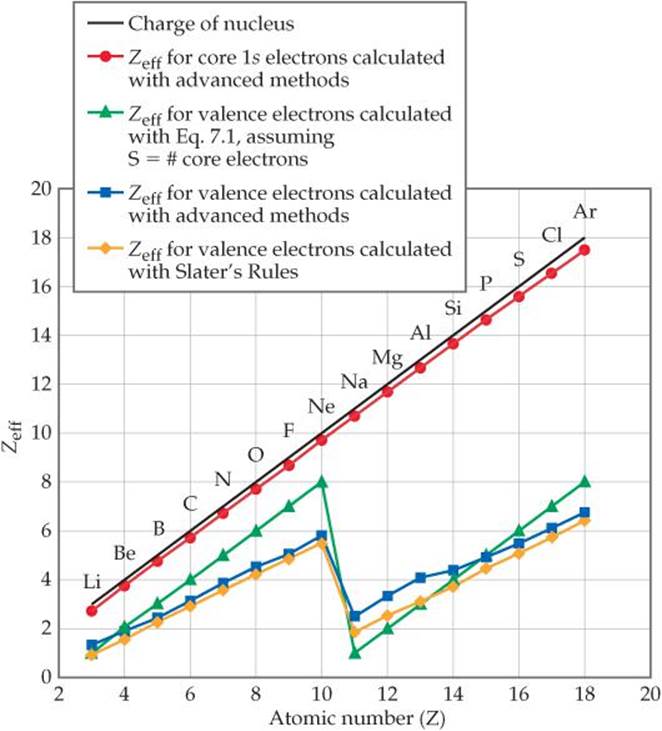
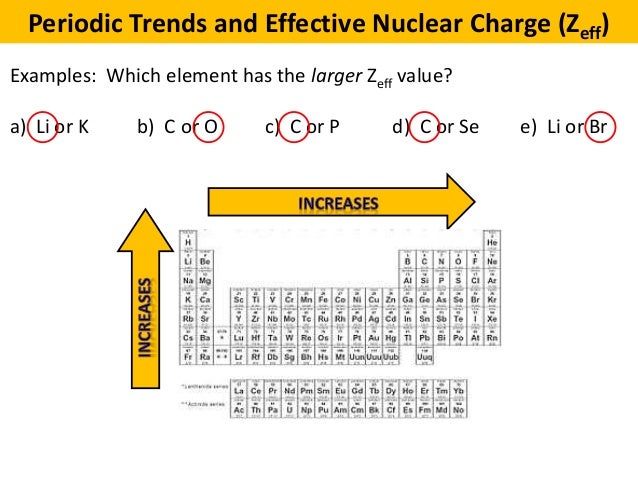
The trend on the periodic table is to increase across a period and increase down a group. Effective nuclear charge refers to the charge felt by the outermost valence electrons of a multi electron atom after the number of shielding electrons that surround the nucleus is taken into account. Therefore using the equation for effective nuclear charge z eff z σ we see that bromine has a greater effective nuclear charge than potassium and that this trend is expected across the whole periodic table.
Bromine has 35 protons. View available hint s atomic size lonization energy electron affinity o all of the answers are correct. Part a which of the following trends is inversely proportional to effective nuclear charge zeff.

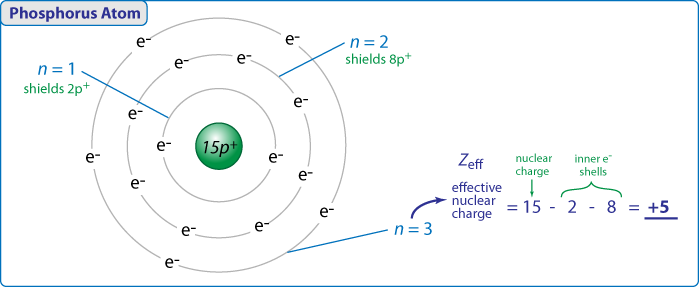
The term effective is used because the shielding effect of negatively charged electrons prevents higher orbital electrons from experiencing the full nuclear charge. The effective nuclear charge often symbolized as z eff or z is the net positive charge experienced by an electron in a multi electron atom. In fact the effective nuclear charge felt by the outermost electrons in cesium is much less than expected 6 rather than 55.
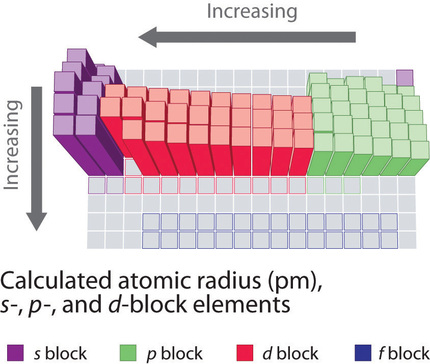
If the outermost electrons in cesium experienced the full nuclear charge of 55 a cesium atom would be very small indeed. That force depends on the effective nuclear charge experienced by the the inner electrons. Slater s rules may be used to calculate an effective nuclear charge.
Measurements indicate the effective nuclear charge experienced by a 2s lithium electron is 0 43 times the charge of the lithium nucleus. The effective nuclear charge often symbolized as or is the net positive charge experienced by an electron in a polyelectronic atom the term effective is used because the shielding effect of negatively charged electrons prevents higher orbital electrons from experiencing the full nuclear charge of the nucleus due to the repelling effect of inner layer electrons.