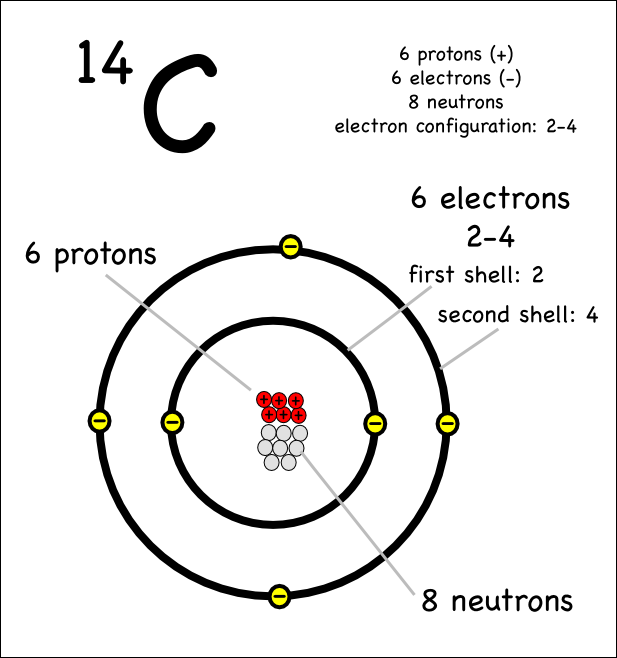
Electron capture a neutron poor nucleus can decay by either positron emission or electron capture ec in which an electron in an inner shell reacts with a proton to produce a neutron. The mass number 11 does not change and the sum of the atomic numbers of the products is 6 the same as the atomic number of the parent carbon 11 nuclide. The atomic number of carbon is 6 which means that every carbon atom has 6 protons so that the neutron numbers of these isotopes are 6 7 and 8 respectively.
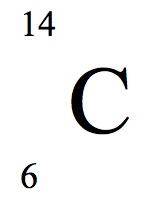
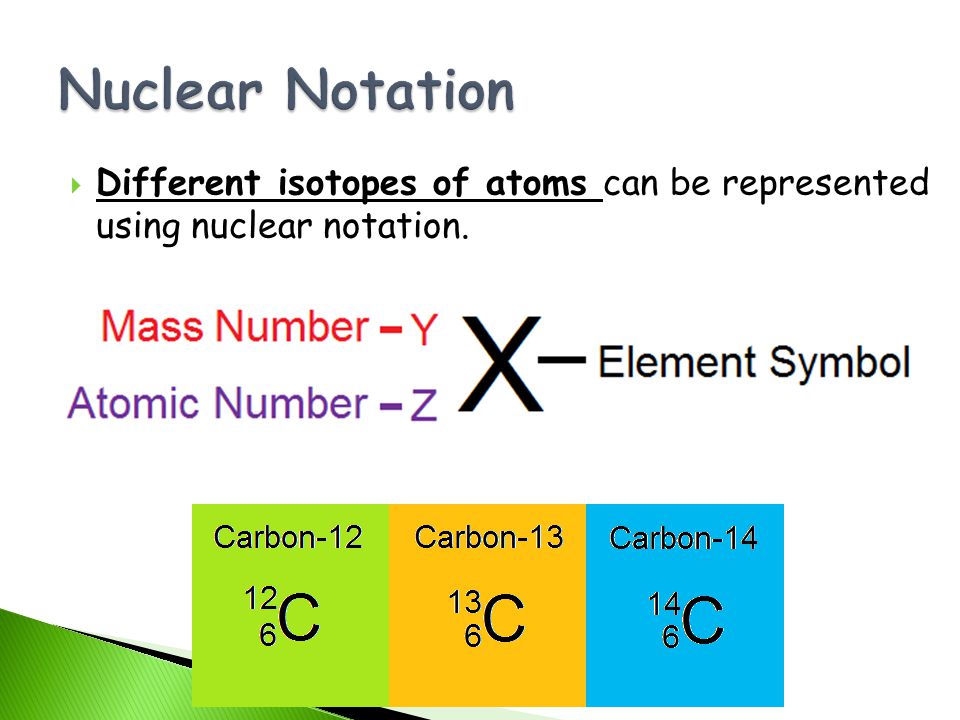
For example carbon 12 carbon 13 and carbon 14 are three isotopes of the element carbon with mass numbers 12 13 and 14 respectively. This radioactive isotope of carbon is called radiocarbon. The imbalance makes carbon 14 a radioisotope with a half life of 5 700 years and an emitter of beta particles.

The nucleus of carbon 14 contains 6 protons and 8 neutrons as opposed to the 6 and 6 found in ordinary carbon 12. Carbon 14 a by product of cosmic rays. Another isotope carbon 14 is useful in studying abnormalities of metabolism that underlie diabetes mellitus gout anemia and acromegaly.
Other articles where carbon 14 is discussed. The mass number is 14. This atom of carbon has 6 protons remember all carbon atoms have 6 protons.
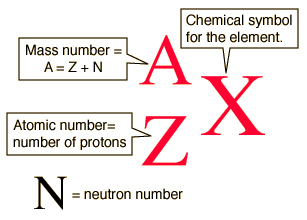
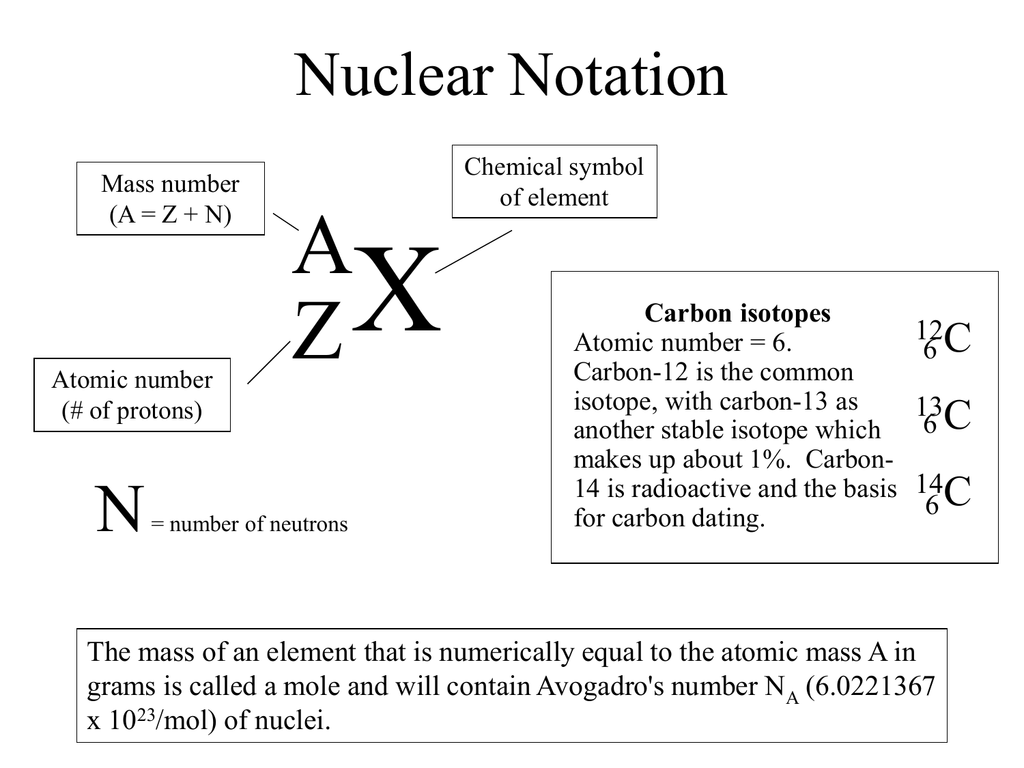
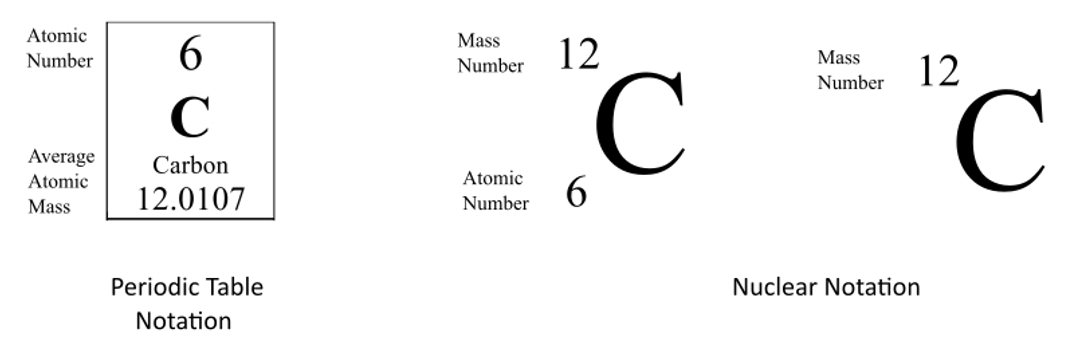
The atomic number is 6. Look at the nuclear notation below for an isotope of carbon. The atomic number z for an element does not change but the mass number a can change as neutrons are added or removed from the nucleus.

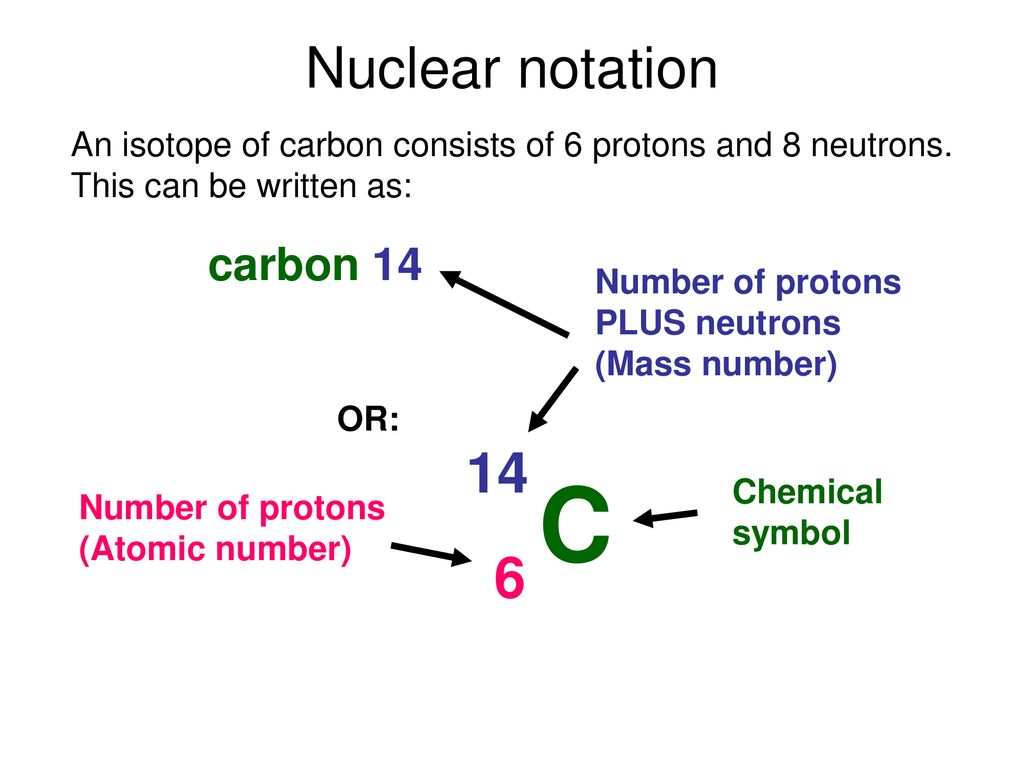
Sometimes the atomic number is omitted from the nuclear notation since we already know carbon has six protons from the atomic number on the periodic table. For nuclear notation the mass number of the isotope goes on top and the atomic number goes on the bottom. Carbon 14 or c 14 8 neutrons 14 6 c.

The mass of an element that is numerically equal to the atomic mass a in grams is called a mole and will contain avogadro s number n a of nuclei.
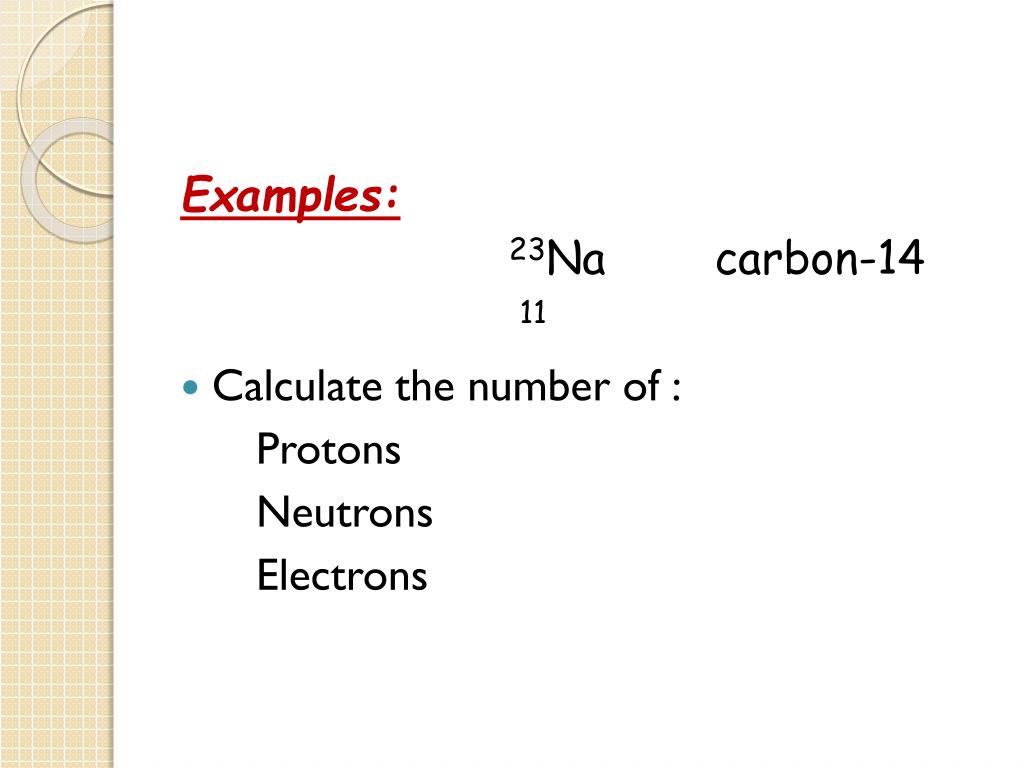
Nuclear notation for carbon 14. Carbon 14 14 c or radiocarbon is a radioactive isotope of carbon with an atomic nucleus containing 6 protons and 8 neutrons its presence in organic materials is the basis of the radiocarbon dating method pioneered by willard libby and colleagues 1949 to date archaeological geological and hydrogeological samples. Carbon 14 was discovered on february 27 1940 by martin kamen and sam. From the periodic table we see that the atomic number number of protons for the element carbon is 6. The name carbon 14 tells us that this isotope s mass number is 14.
The chemical symbol for carbon is c. Now write the isotopic notation for carbon 14. 6 14 c we can determine the number of neutrons as 14 6 8 neutrons. Carbon 12 is the common isotope with carbon 13 as another stable isotope which makes up about 1.
Carbon 14 is radioactive and the basis for carbon dating.

Carbon 14 is radioactive and the basis for carbon dating. Carbon 12 is the common isotope with carbon 13 as another stable isotope which makes up about 1. 6 14 c we can determine the number of neutrons as 14 6 8 neutrons.
Now write the isotopic notation for carbon 14. The chemical symbol for carbon is c. The name carbon 14 tells us that this isotope s mass number is 14.

From the periodic table we see that the atomic number number of protons for the element carbon is 6. Carbon 14 was discovered on february 27 1940 by martin kamen and sam. Carbon 14 14 c or radiocarbon is a radioactive isotope of carbon with an atomic nucleus containing 6 protons and 8 neutrons its presence in organic materials is the basis of the radiocarbon dating method pioneered by willard libby and colleagues 1949 to date archaeological geological and hydrogeological samples.