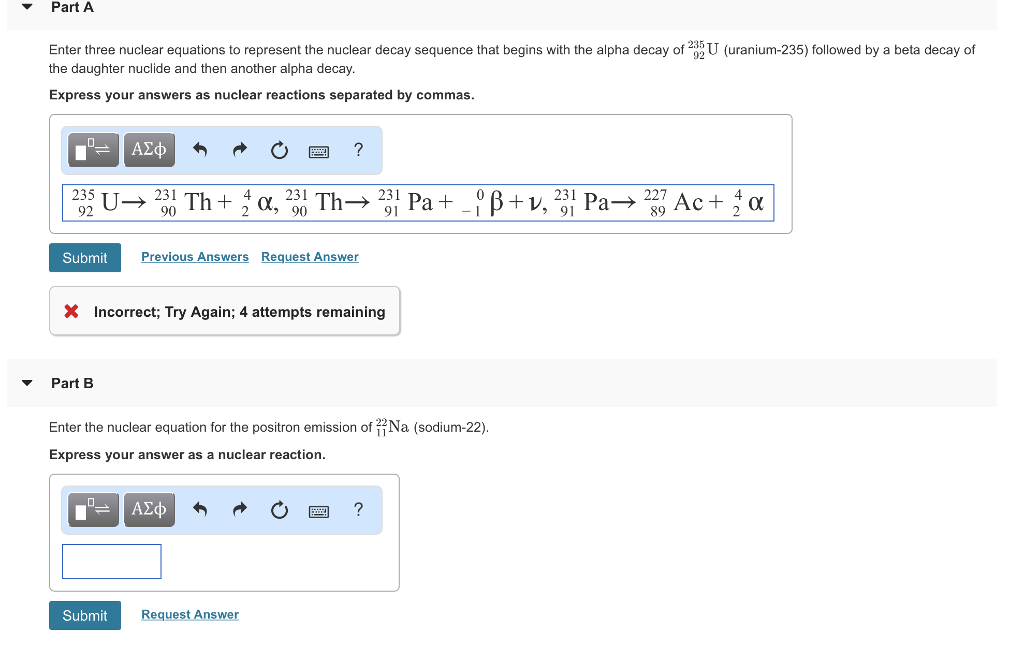
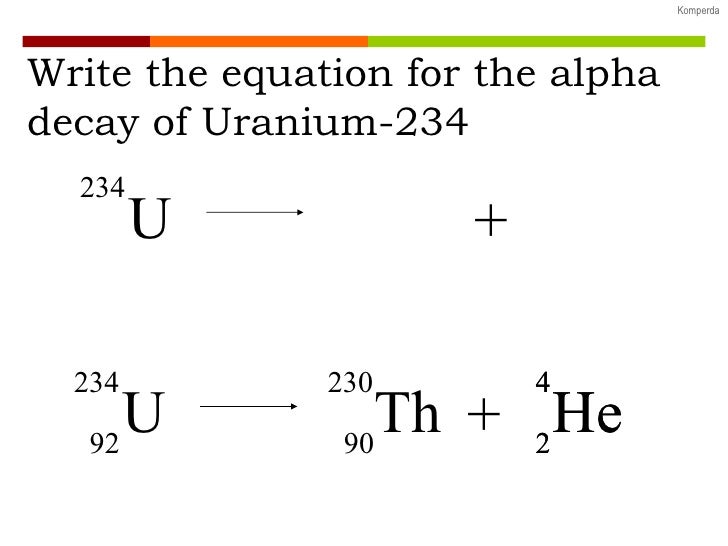
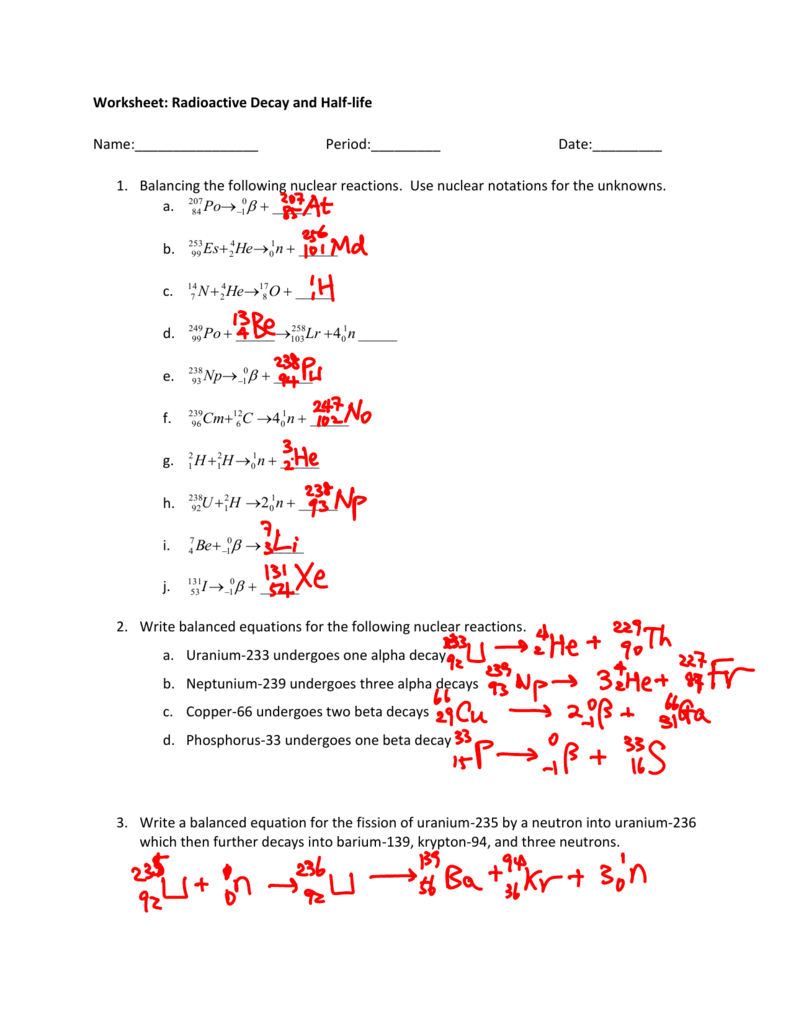
235u also decays by spontaneous fission but the results are somewhat. 90231th 24he representing the alpha particle as a helium nucleus. The equation for the alpha decay of 235u is.

A fourth series the decay of neptunium 237 to bismuth 209 in 11 steps is known to have occurred on the primitive earth. The decay of uranium 235 to lead 207 in 11 steps and thorium 232 to lead 208 in 10 steps. Although a radioactive decay series can be written for almost any isotope with z 85 only two others occur naturally.

Its specific activity is very low 2 2 10 6 ci g. Uranium 235 occasionally decays by spontaneous fission with very low probability of 0 0000000072. Half life of uranium 235 is 7 x 10 8 years.
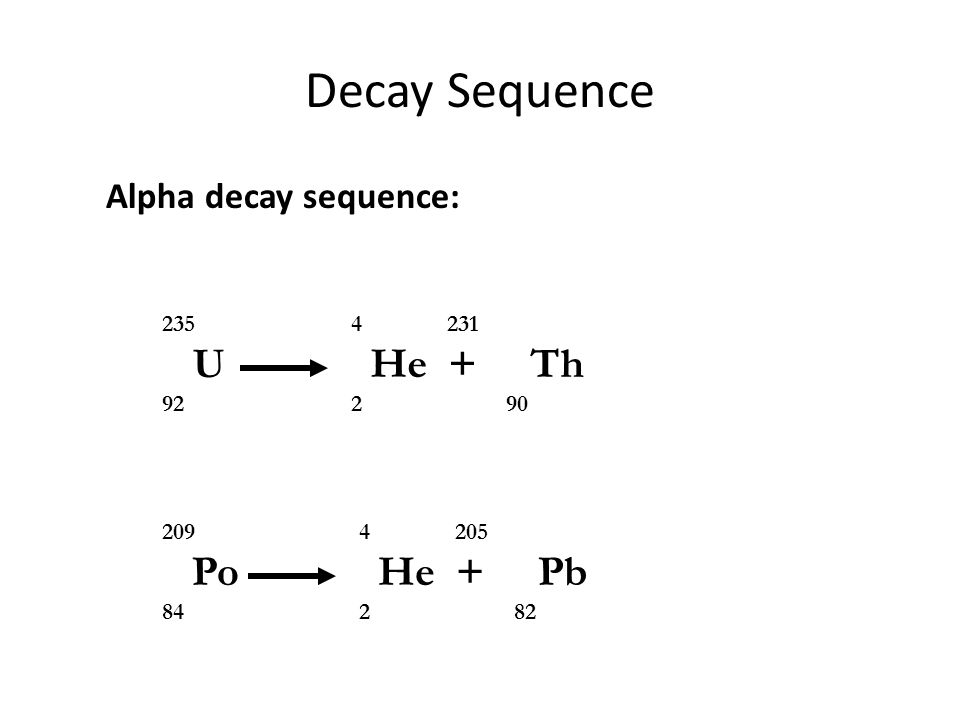
Uranium 235 decays via alpha decay by way of thorium 231 into 231 pa. In the uranium 235 natural decay series the uranium 235 ini. Problem 48ep from chapter 11.

Study guide with selected solutions for stoker s general organic and biological chemistry 6th edition edit edition. If the reaction will sustain itself it is said to be critical and the mass of u 235 required to produced the critical condition is said to be a critical mass. Uranium 235 chain reaction if an least one neutron from u 235 fission strikes another nucleus and causes it to fission then the chain reaction will continue.
235u also decays by spontaneous fission but the results are somewhat. 92235u 90231th 24he representing the alpha particle as a helium nucleus. The equation for the alpha decay of 235u is.
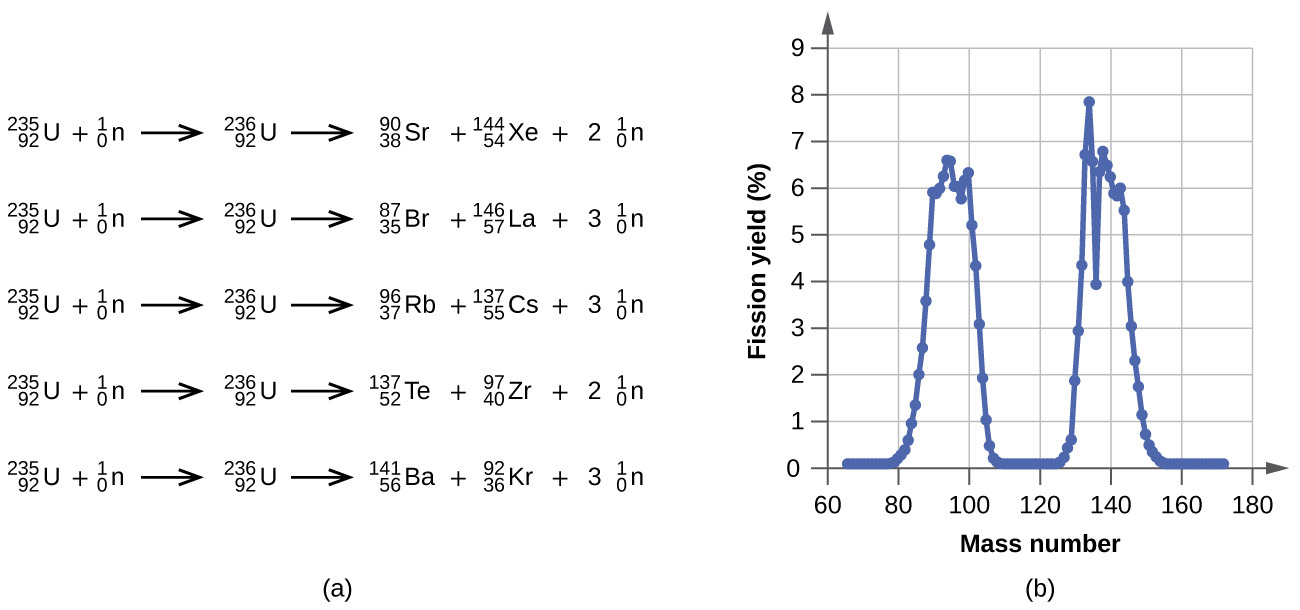
One of these fission reactions produces barium krypton and 3 neutrons.
Nuclear decay equation for uranium 235. Following is one decay equation for the alpha decay of this isotope. 92 235 u 90 231 th 2 4 he. Here the he is representing one alpha α particle. Uranium 235 undergoes spontaneous fission during radioactive decay.
However no standard equation can represent this reaction as its results are quite unpredictable. Uranium 235 235 u is an isotope of uranium making up about 0 72 of natural uranium unlike the predominant isotope uranium 238 it is fissile i e it can sustain a fission chain reaction it is the only fissile isotope that is primordial and found in relatively significant quantities in nature. Uranium 235 has a half life of 703 8 million years. It was discovered in 1935 by arthur jeffrey.
Uranium 235 is a fissile isotope and its fission cross section for thermal neutrons is about 585 barns for 0 0253 ev neutron. For fast neutrons its fission cross section is on the order of barns most of absorption reactions result in fission reaction but a minority results in radiative capture forming 236 u. The cross section for radiative capture for thermal neutrons. Write a nuclear reaction equation for the decay of thorium 232 by alpha emission followed by beta emission.
When an atom of uranium 235 captures a neutron the product uranium 236 is unstable and splits into two lighter nuclides.
When an atom of uranium 235 captures a neutron the product uranium 236 is unstable and splits into two lighter nuclides. Write a nuclear reaction equation for the decay of thorium 232 by alpha emission followed by beta emission. The cross section for radiative capture for thermal neutrons.
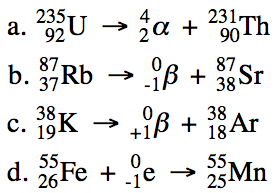
For fast neutrons its fission cross section is on the order of barns most of absorption reactions result in fission reaction but a minority results in radiative capture forming 236 u. Uranium 235 is a fissile isotope and its fission cross section for thermal neutrons is about 585 barns for 0 0253 ev neutron. It was discovered in 1935 by arthur jeffrey.

Uranium 235 has a half life of 703 8 million years. Uranium 235 235 u is an isotope of uranium making up about 0 72 of natural uranium unlike the predominant isotope uranium 238 it is fissile i e it can sustain a fission chain reaction it is the only fissile isotope that is primordial and found in relatively significant quantities in nature. However no standard equation can represent this reaction as its results are quite unpredictable.

Uranium 235 undergoes spontaneous fission during radioactive decay. Here the he is representing one alpha α particle. 92 235 u 90 231 th 2 4 he.
Following is one decay equation for the alpha decay of this isotope.