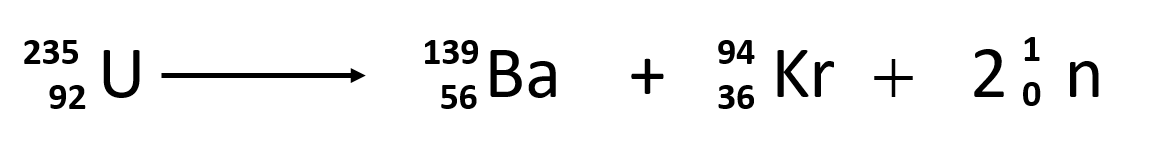The reactors use nuclear fuel most commonly uranium 235 and plutonium 239. A nuclear reactor is a piece of equipment where nuclear chain reactions can be controlled and sustained. The energy released from nuclear fission can be harnessed to make electricity with a nuclear reactor.
It was discovered in 1935 by arthur jeffrey. Uranium 235 has a half life of 703 8 million years. Uranium 235 235 u is an isotope of uranium making up about 0 72 of natural uranium unlike the predominant isotope uranium 238 it is fissile i e it can sustain a fission chain reaction it is the only fissile isotope that is primordial and found in relatively significant quantities in nature.
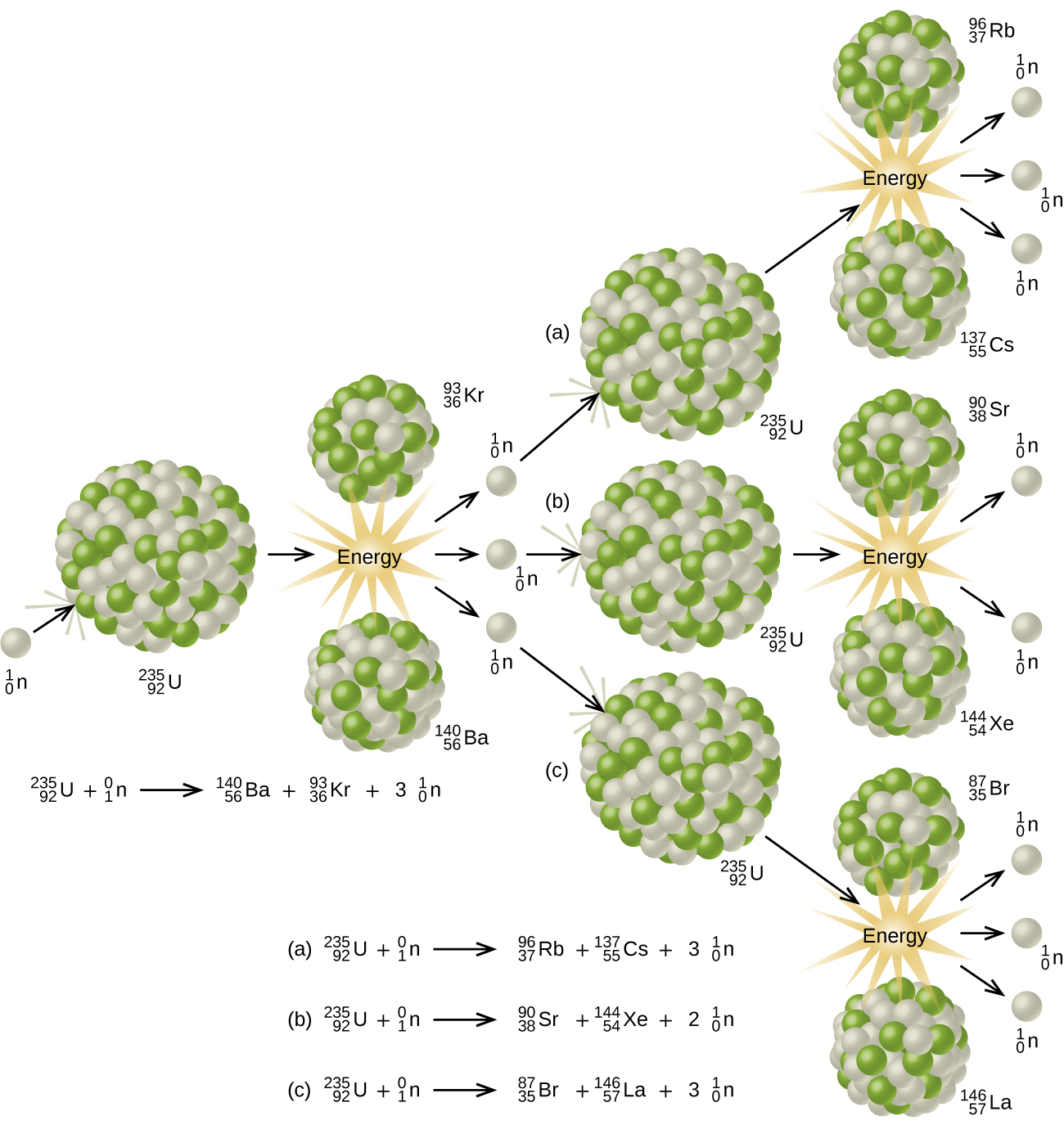
Uranium 235 is the only fissile radioactive isotope which is a primordial nuclide existing in the nature in its present form since before the creation of earth. Uranium 235 is a naturally occurring isotope of uranium metal it is the only fissile uranium isotope being able to sustain nuclear fission. These fuels break apart into a bimodal range of chemical elements with atomic masses centering near 95 and 135 u fission products.
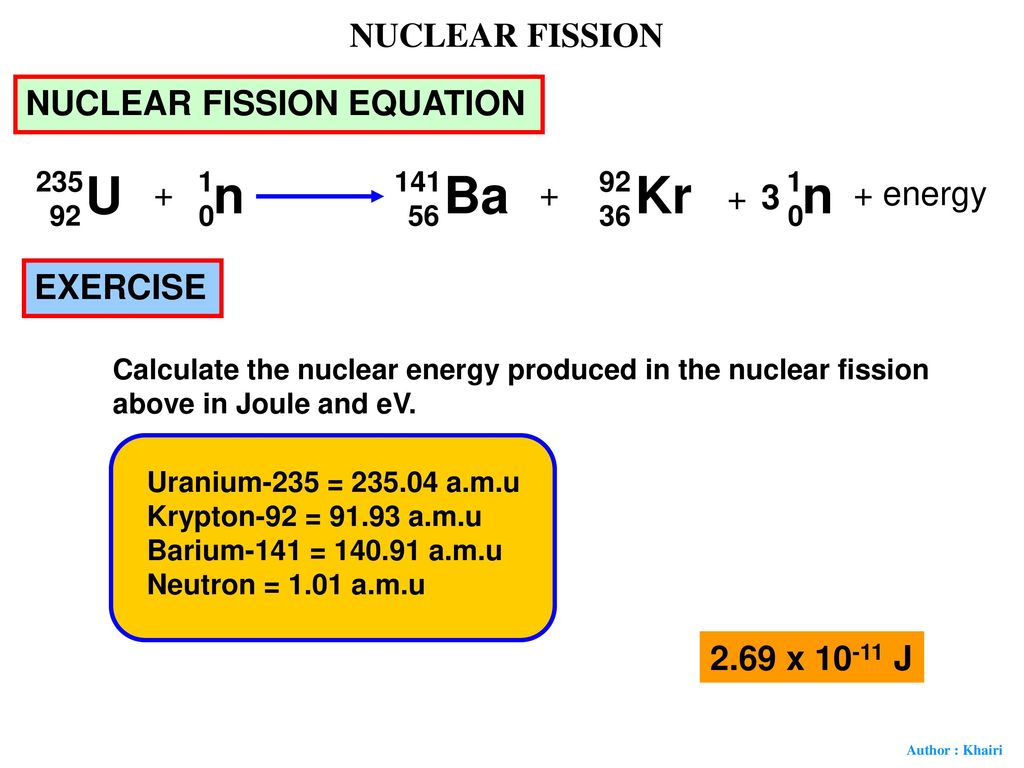
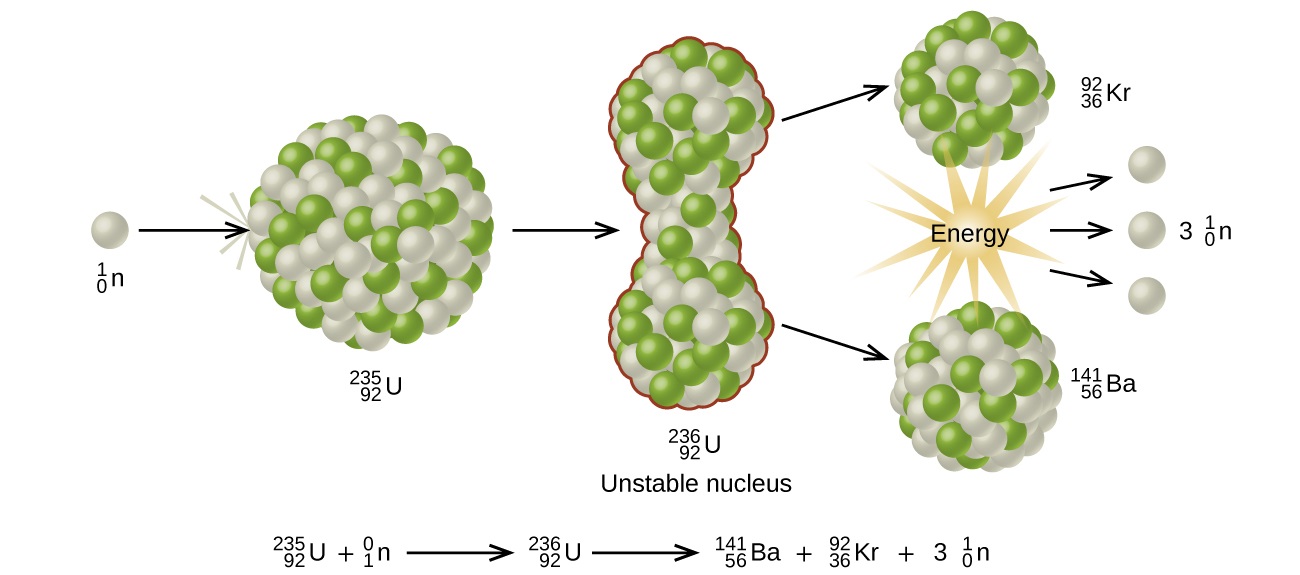
The most common nuclear fuels are 235 u the isotope of uranium with mass number 235 and of use in nuclear reactors and 239 pu the isotope of plutonium with mass number 239. A nuclear equation showing a typical fission of uranium 235 is shown below. As the nucleus breaks apart a significant amount of energy is also released.
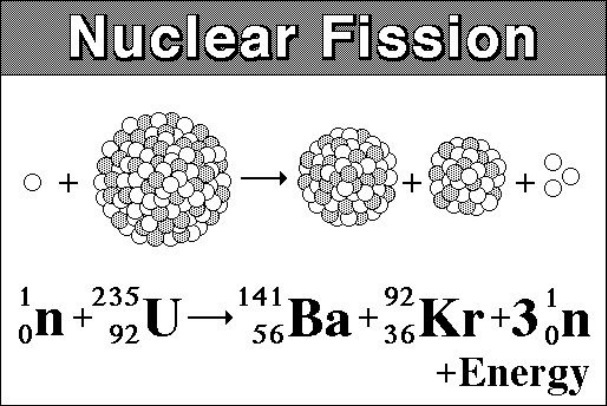
The unstable nucleus instantaneously breaks apart undergoes fission to form lighter elements and to release additional free neutrons. The nuclear fission of uranium 235 is shown in the following equation. The collision results in nuclear fission.

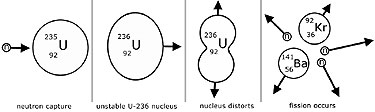
Scientists usually accomplish this task for some controlled nuclear reactions by bombarding a large isotope with a second smaller one commonly a neutron. Nuclear fission occurs when a larger isotope breaks apart into two or more elements. If the reaction will sustain itself it is said to be critical and the mass of u 235 required to produced the critical condition is said to be a critical mass.

Uranium 235 chain reaction if an least one neutron from u 235 fission strikes another nucleus and causes it to fission then the chain reaction will continue.
Nuclear fission equation of uranium 235. Uranium 235 is a fissile isotope and its fission cross section for thermal neutrons is about 585 barns for 0 0253 ev neutron. For fast neutrons its fission cross section is on the order of barns most of absorption reactions result in fission reaction but a minority results in radiative capture forming 236 u. The cross section for radiative capture for thermal neutrons. Scientists usually accomplished this task by bombarding a large isotope with a second smaller one commonly a neutron.
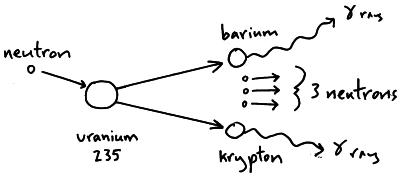
The collision caused the larger isotope to break apart into two or more elements which is called nuclear fission. Figure 1 shows the equation for the nuclear fission of uranium 235. Nuclear fission is the splitting of a large atomic nucleus into smaller nuclei. In a nuclear reactor a neutron is absorbed into a nucleus typically uranium 235.
This causes the nucleus to.
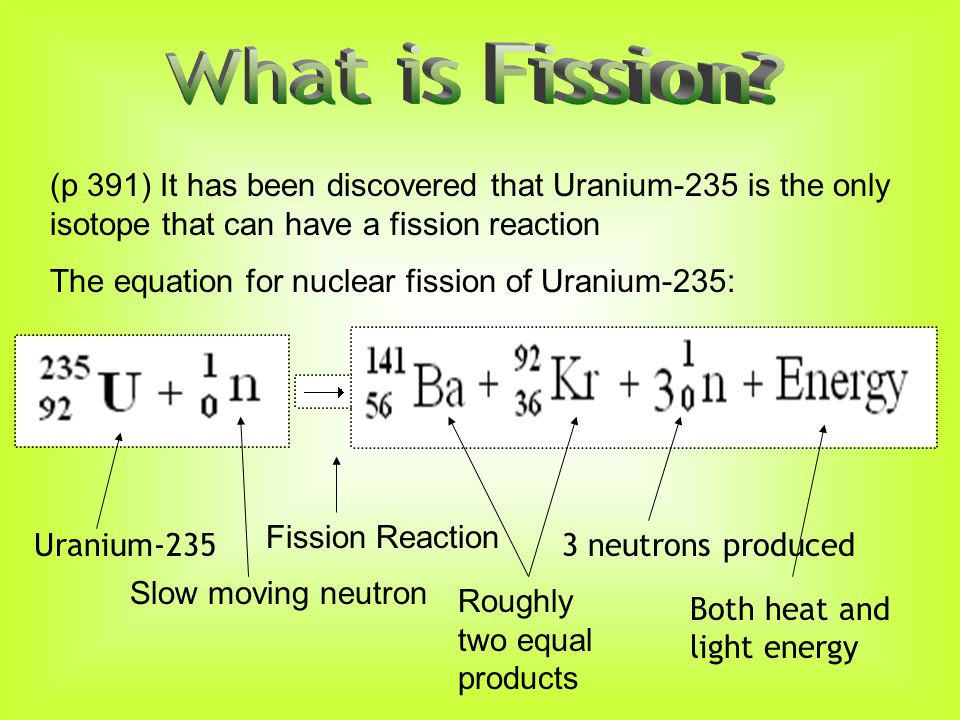
This causes the nucleus to. In a nuclear reactor a neutron is absorbed into a nucleus typically uranium 235. Nuclear fission is the splitting of a large atomic nucleus into smaller nuclei.

Figure 1 shows the equation for the nuclear fission of uranium 235. The collision caused the larger isotope to break apart into two or more elements which is called nuclear fission. Scientists usually accomplished this task by bombarding a large isotope with a second smaller one commonly a neutron.

The cross section for radiative capture for thermal neutrons. For fast neutrons its fission cross section is on the order of barns most of absorption reactions result in fission reaction but a minority results in radiative capture forming 236 u. Uranium 235 is a fissile isotope and its fission cross section for thermal neutrons is about 585 barns for 0 0253 ev neutron.