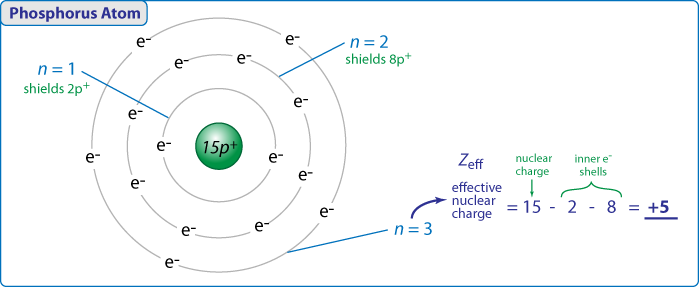
Follows the same trend as the first ionization. Outermost electrons known as valence electrons are furthest from the nucleus. 1s 2s experience the greatest effective nuclear charge experience the least shielding.

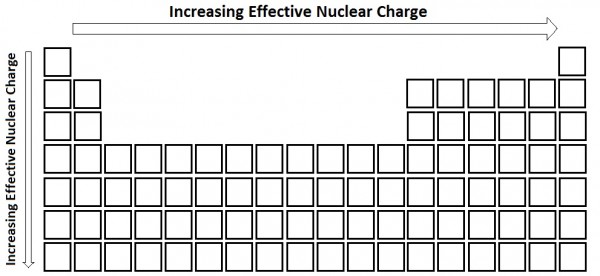
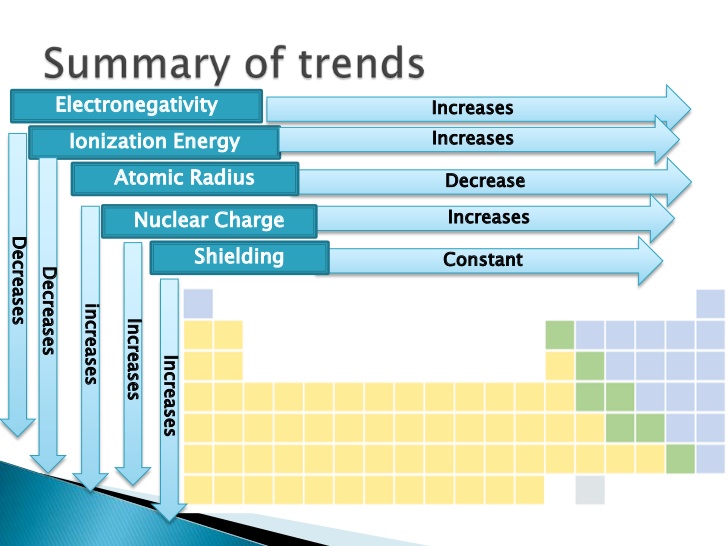
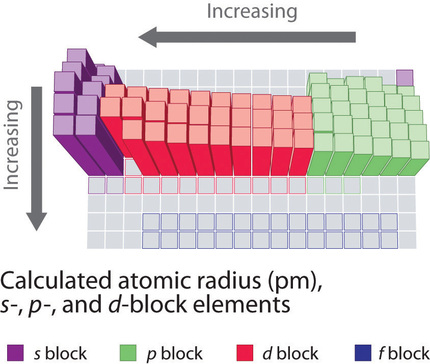
Moving across the periodic table increases the effective nuclear charge and therefore increases the force of attraction. The size of an atom decreases as we move across the period because the electrons get added to the same shell and the nuclear charge keeps on increasing. It decreases because the effective nuclear charge decreases while moving left to right.

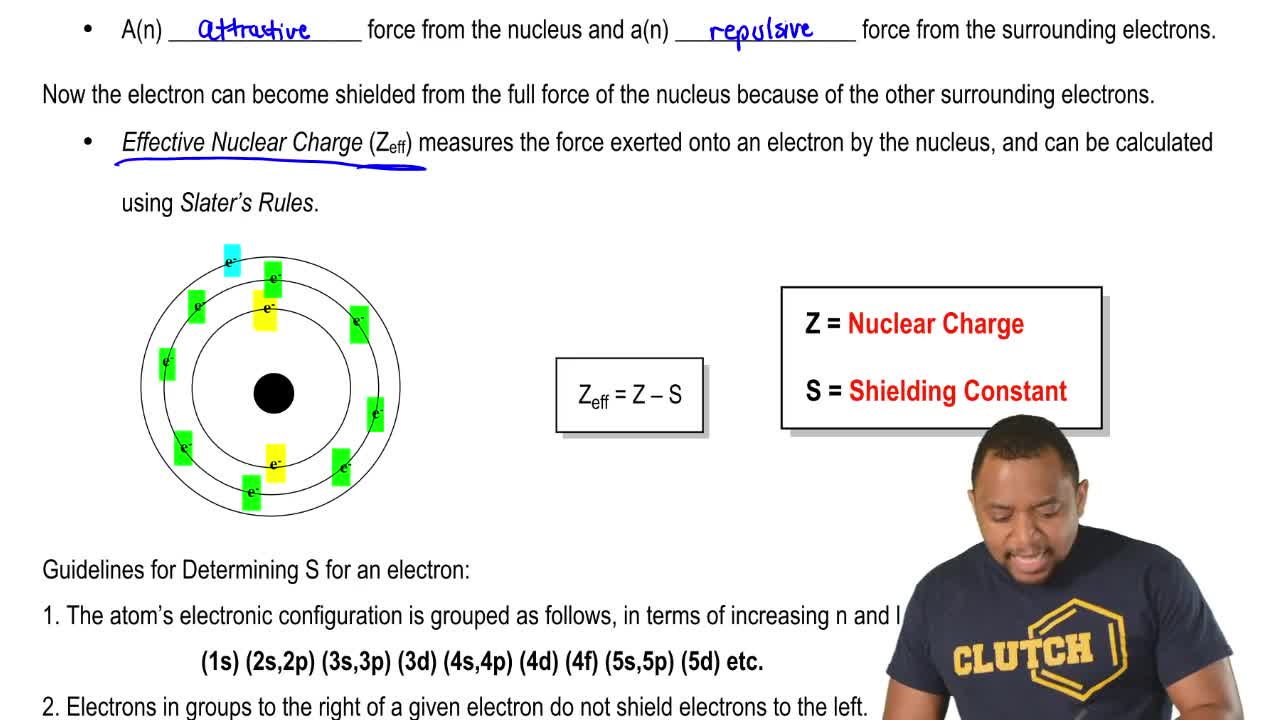
Which of the following best explains the trend in ionization energy across a period. The trend on the periodic table is to increase across a period and increase down a group. Effective nuclear charge refers to the charge felt by the outermost valence electrons of a multi electron atom after the number of shielding electrons that surround the nucleus is taken into account.

Therefore using the equation for effective nuclear charge z eff z σ we see that bromine has a greater effective nuclear charge than potassium and that this trend is expected across the whole periodic table. Bromine has 35 protons. Additionally because chlorine is in the same group as bromine but is higher up on the periodic table it has a greater effective nuclear charge making it.
Because chlorine is in the same period as phosphorus and sodium but has the most protons in its shell the most right within the same period it has the greatest effective nuclear charge. This helps us predict periodic trends. This helps us predict periodic trends.

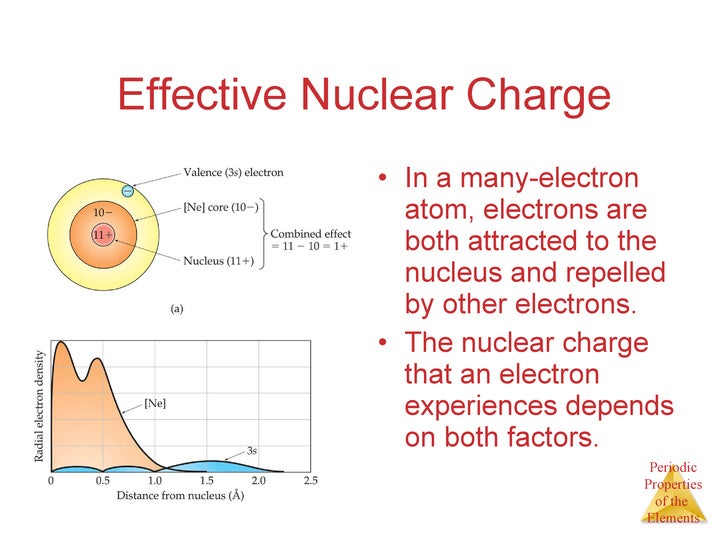
We learned that effective nuclear charge is the positive charge felt by the outermost electrons in an atom. In fact the effective nuclear charge felt by the outermost electrons in cesium is much less than expected 6 rather than 55. If the outermost electrons in cesium experienced the full nuclear charge of 55 a cesium atom would be very small indeed.

That force depends on the effective nuclear charge experienced by the the inner electrons.
Greatest effective nuclear charge trend. The effective nuclear charge often symbolized as or is the net positive charge experienced by an electron in a polyelectronic atom the term effective is used because the shielding effect of negatively charged electrons prevents higher orbital electrons from experiencing the full nuclear charge of the nucleus due to the repelling effect of inner layer electrons. Effective nuclear charge z eff is the reduced nuclear charge experienced by an electron. This trend is best illustrated by inspection of figure pageindex 3. The trend depends on shell and subshell.
Periods 1 3 s and p only. As we go across the table in periods 1 3 the shell stays constant as z increases and. The effective nuclear charge often symbolized as z eff or z is the net positive charge experienced by an electron in a multi electron atom. The term effective is used because the shielding effect of negatively charged electrons prevents higher orbital electrons from experiencing the full nuclear charge.

The term effective is used because the shielding effect of negatively charged electrons prevents higher orbital electrons from experiencing the full nuclear charge. The effective nuclear charge often symbolized as z eff or z is the net positive charge experienced by an electron in a multi electron atom. As we go across the table in periods 1 3 the shell stays constant as z increases and.

Periods 1 3 s and p only. The trend depends on shell and subshell. This trend is best illustrated by inspection of figure pageindex 3.

Effective nuclear charge z eff is the reduced nuclear charge experienced by an electron. The effective nuclear charge often symbolized as or is the net positive charge experienced by an electron in a polyelectronic atom the term effective is used because the shielding effect of negatively charged electrons prevents higher orbital electrons from experiencing the full nuclear charge of the nucleus due to the repelling effect of inner layer electrons.