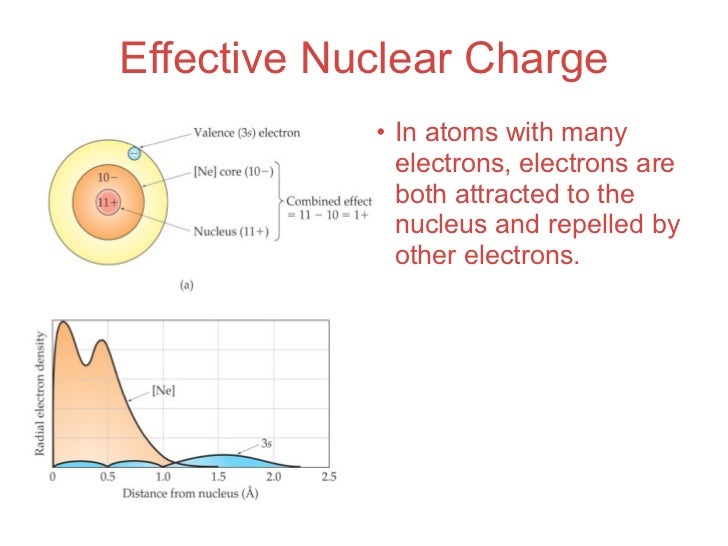
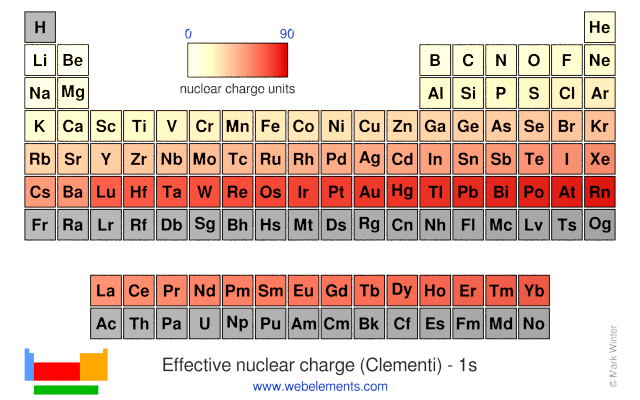
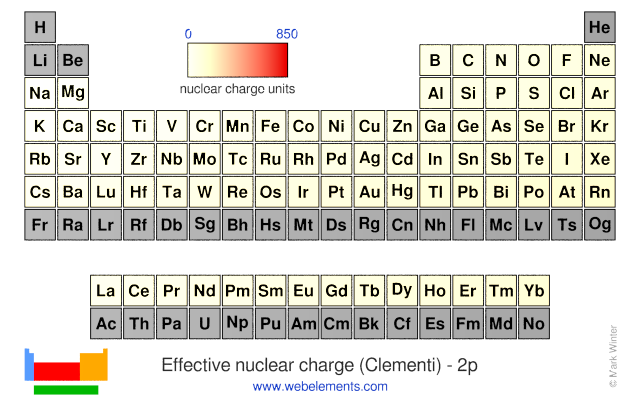
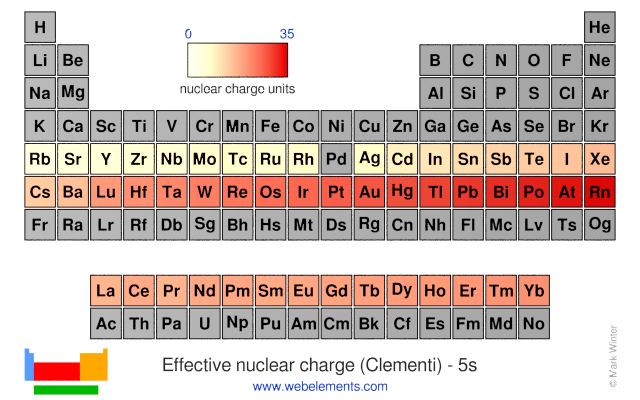
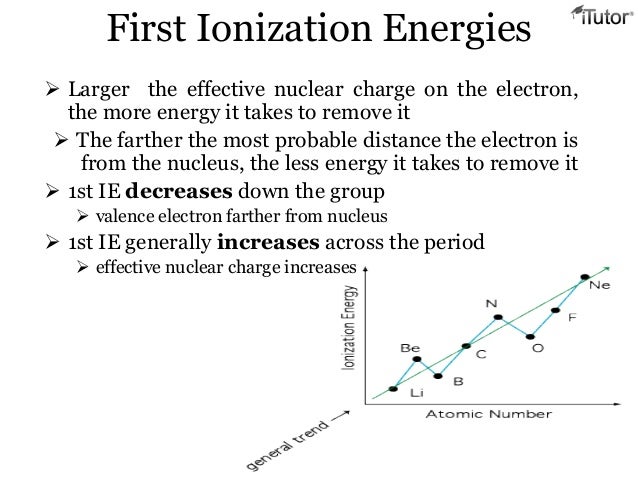
Effective nuclear charge is the net charge that an outer shell electron experiences in an atom. Nuclear charge is the total charge of the nucleus. Difference between nuclear charge and effective nuclear charge definition.

In this topic we are going to discuss the effective nuclear charge and how to calculate it. Also the electron or multi electron takes into account the number of shielding electrons that surrounds the nucleus. Effective nuclear charge refers to the charge that the outermost valance electron have.

Introduction to effective nuclear charge. The term effective is used because the shielding effect of negatively charged electrons prevents higher orbital electrons from experiencing the full nuclear charge. The effective nuclear charge often symbolized as z eff or z is the net positive charge experienced by an electron in a multi electron atom.
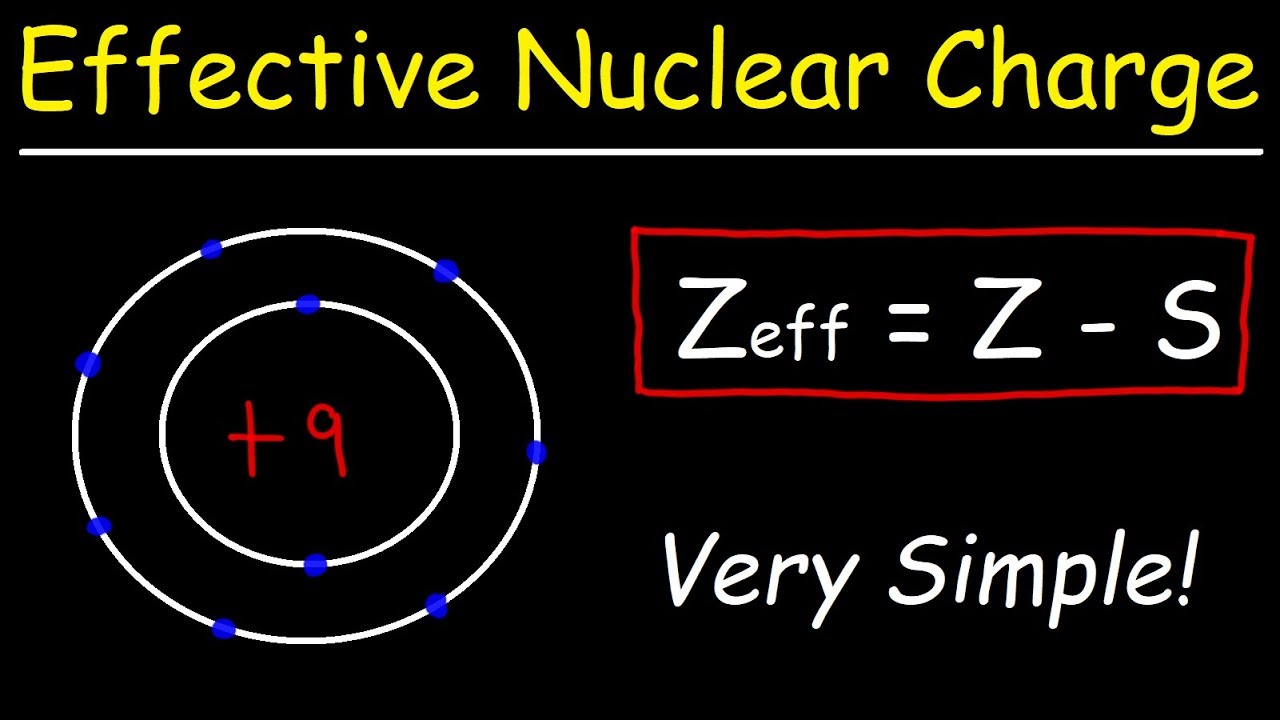
The term effective is used because the shielding effect of negatively charged electrons prevents higher orbital electrons from experiencing the full nuclear charge by the repelling effect of inner layer electrons. The effective nuclear charge often symbolized as or is the net positive charge experienced by an electron in a multi electron atom. Those d orbitals within a set with lobes directed along the x y and z axes.
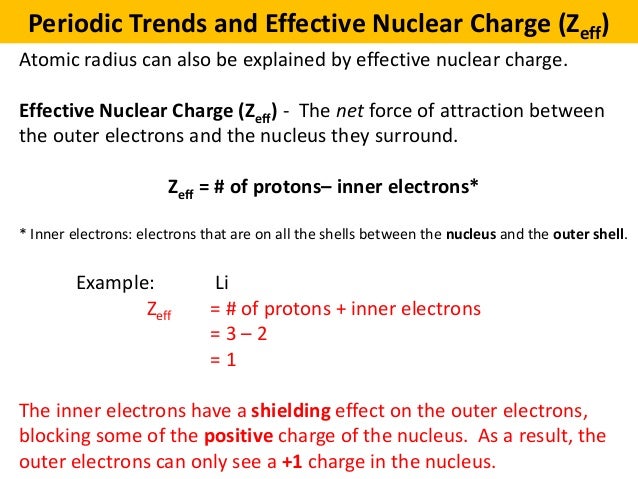
Set of dx2 y2 and dz2 orbitals. The actual nuclear charge minus the effects of shielding due to inner shell electrons. Definition of effective nuclear charge the nuclear charge experienced by the outermost electrons of an atom.

The effective nuclear charge experienced by the outer shell electron is. The effective nuclear charge is the net positive charge experienced by an electron in a multi electron atom the term effective is used because the shielding effect of negatively charged electrons prevents higher orbital electrons from experiencing the full nuclear charge by the repelling effect of inner layer electrons. The effective nuclear charge often symbolized as or is the net positive charge experienced by an electron in a polyelectronic atom the term effective is used because the shielding effect of negatively charged electrons prevents higher orbital electrons from experiencing the full nuclear charge of the nucleus due to the repelling effect of inner layer electrons.

Slater s rules may be used to calculate an effective nuclear charge.
Effective nuclear charge meaning. Measurements indicate the effective nuclear charge experienced by a 2s lithium electron is 0 43 times the charge of the lithium nucleus.
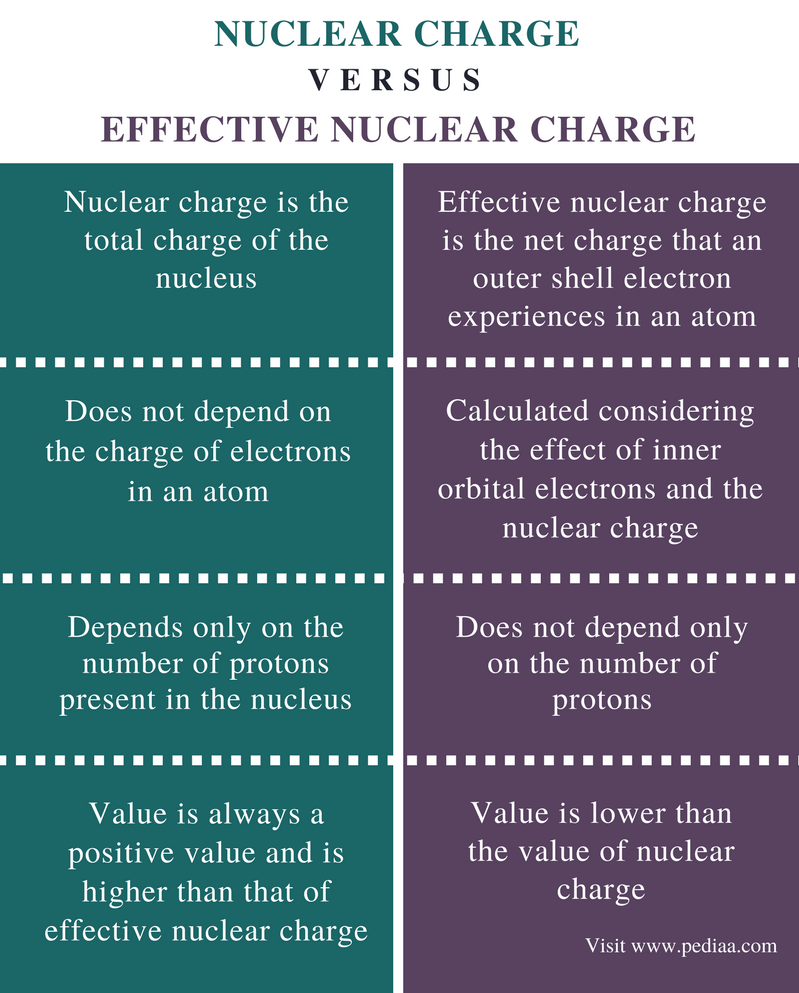
Measurements indicate the effective nuclear charge experienced by a 2s lithium electron is 0 43 times the charge of the lithium nucleus.